RT-LAMP
protocol with commercial reagents
RT-LAMP with commercially bought reagents is the easiest and fastest way to get started with SARS-CoV-2 detection with reverse transcription loop mediated isothermal amplification. This page includes the protocol, material list and a benchmarking assay that you can use to confirm your LAMP reactions are effective. See the limit of detection assay for our data on the sensitivity and specificity of this method.
Some things to keep in mind before getting started:
- SARS-CoV-2 is a highly contagious virus and requires personal protection with appropriate safety standards. Please follow Biosafety level guidelines outlined by the CDC or your local authorities.
- RT-LAMP reactions are incompatible with samples in collection media containing more than 1 mM of metal chelators such as EDTA or EGTA and with sample collection media containing chaotropic reagents such as guanidium thiocyanate and guanidium hydrochloride.
- Separate working areas for sample preparation, reaction set-up and analysis are highly recommended. Clean work surfaces and pipettes with 5-10% bleach solution.
- Do not open RT-LAMP reactions in order to prevent carry-over cross contamination leading to false positives. The non-colorimetric master mix from NEB (E1700L) does not contain dUTP/thermolabile UDG to prevent cross-contamination; we recommend to add these two components to the reaction mix to lower the risk of cross-reaction contamination.
- 96-well plates must be properly sealed. This is absolutely crucial to prevent cross-well contamination. Proper plate seals (e.g. tight sealing plastic covers or aluminium seals), a proper plate-roller (e.g. BioRad) or even better a PCR plate sealer (e.g. BioRad PX1 PCR plate sealer) can help prevent cross-well contamination.
- Store reaction components at the appropriate temperatures outline by the manufacturer. When thawing the NEB WarmStart LAMP kit, we advise thawing at room temperature for 10 minutes with vortex mixing for 5 seconds every 5 minutes. This ensures that any Magnesium sulfate precipitate goes into solution, which is essential for proper HNB colorimetric LAMP detection and proper performance. Check Mastermix after thawing to ensure no precipitate is visible.
- Colorimetric and fluorescent detection can be performed in the same reaction by simple addition of both dyes (the colorimetric HNB dye and the fluorescent LAMP dye that is provided with the kit).
- When working with crude samples lysed in QuickExtract, keep samples on ice whenever possible. While we have observed complete inactivation of nucleases after 5 minutes at 95°C, we found considerable RNase activity in biological sample types such as saliva and gargle. Due to the FBS content in Universal Transport Media (UTM), nasopharyngeal swabs in this medium may also contain RNases.
- RT-LAMP uses six primers, two inner loop primers (FIP, BIP), loop primers (LF, LB) and outer bumping primers (F3, B3). A reaction contains all six primers if a single amplicon is tested.
- A positive control amplicon detecting the human ACTB gene can be used to confirm the presence of human nucleic acid sample material in the sample, and the absence of general reaction-inhibiting substances in the sample.
- A crucial component of every experiment and testing procedure is the selection of proper controls. To verify that your reaction mastermix works in the given conditions for the detection of the SARS-CoV-2 genome, we recommend using an inactivated qPCR positive patient sample, synthetic SARS-CoV-2 RNA or plasmid DNA containing the Orf1ab region. Positive controls should have a concentration exceeding 100 copies/µl. A verified qPCR negative sample is a proper choice for a negative control.
Materials
LAMP primer mix 10X
LAMP requires six primers for optimal functioning. That is six short DNA oligonucleotides to synthesise or order, the names are F3, B3, LF, LB, FIP and BIP. For the ease of use, one can mix those in a pre-determined ratio to make a 10X primer mix to use with LAMP. You can make this mix with As1 primers against SARS-CoV-2 genome or against human ACTB transcript. Aliquot and label the finished primer mix with the name, date and name of the person who prepared it. We encourage to test every primer mix you receive for activity against the target template, especially when ordering from vendors you don’t have prior experience with.
- Store primer mixes at -20 ºC.
- Order oligonucleotides from reputable companies specialising in DNA oligonucleotide synthesis.
- When ordering oligos, choose "standard desalting" as the purity. We recommend ordering primers resuspended in 100 μM concentration. You can order yours lyophilised and then resuspend them in nuclease-free water according to the supplier's instructions.
- This procedure is applicable for every LAMP primer set we recommend here.
Materials
SARS-COV-2 PRIMER SEQUENCES – As1 primers:
As1_F3 CGGTGGACAAATTGTCAC
As1_B3 CTTCTCTGGATTTAACACACTT
As1_LF TTACAAGCTTAAAGAATGTCTGAACACT
As1_LB TTGAATTTAGGTGAAACATTTGTCACG
As1_FIP TCAGCACACAAAGCCAAAAATTTATCTGTGCAAAGGAAATTAAGGAG
As1_BIP TATTGGTGGAGCTAAACTTAAAGCCCTGTACAATCCCTTTGAGTG
POSITIVE CONTROL PRIMER SEQUENCES – ACTB primers:
ACTB-F3 AGTACCCCATCGAGCACG
ACTB-B3 AGCCTGGATAGCAACGTACA
ACTB-FIP GAGCCACACGCAGCTCATTGTATCACCAACTGGGACGACA
ACTB-BIP CTGAACCCCAAGGCCAACCGGCTGGGGTGTTGAAGGTC
ACTB-LoopF TGTGGTGCCAGATTTTCTCCA
ACTB-LoopB CGAGAAGATGACCCAGATCATGT
procedure
- Prepare your ordered oligonucleotides. If they are not resuspended, resuspend them in nuclease-free water for 100 μM stock concentration.
- In a clean and template-free workspace, such as in a PCR hood, mix the oligonucleotides in the order outlined by the table below. Make sure to add the components from largest volume to the smallest volume.
Oligo name Add for 100 μl of mix F3 2 μl B3 2 μl LF 4 μl LB 4 μl FIP 16 μl BIP 16 μl nuclease-free water 56 μl - Mix the finished primer mix by thoroughly pipetting up and down or by vortexing and briefly spinning down in a microfuge.
- Distribute the finished primer mix into Eppendorf tubes into conveniently sized aliquots, label the tubes and store at -20 ºC.
HNB solution preparation
In RT-LAMP assays, we use hydroxynaphtol blue as the dye that lends the reaction its easy and reliable colour readout. HNB is a dye commonly used in metallometric titrations for water purity assessment, as it changes colour from blue to pink in the presence of the magnesium cations. It is critical to use the trisodium salt of hydroxynaphtol blue. Using 3 mM stock solution of HNB is recommended for its stability and ease of pipetting even for a small number of reactions.
- Store finished HNB solution at 4 ºC.
- Buy HNB trisodium salt powder from reputable chemical suppliers. Buy the highest purity grade available.
- HNB solution is stable for several months in the fridge.
procedure
- Weigh out 0.186 g of hydroxynaphtol blue trisodium salt powder on an analytical scale.
- Dissolve the powder in 100 millilitres of nuclease free water. Make sure that every bit of the powder is dissolved in the solution to get a precise 3 mM concentration of the dye.
- Filter the solution through a 0.22 µm sterile filter to make a sterile solution.
- Store the 100 ml of 3 mM solution in the fridge at 4 ºC.
LAMP reagents
Commercial RT-LAMP assays are powered by enzymes that are produced by specialised companies. We found the LAMP kits from NEB to be very powerful in terms of performance and sensitivity, when used with our protocol. Therefore, we recommend purchasing the following reagents to anyone trying to get LAMP working in their settings as soon as possible.
- Store the individual reagents according to the manufacturer's recommendation.
Materials
- QuickExtract DNA solution 50ml (Lucigen QE09050), store at -20°C
- WarmStart LAMP Kit (DNA & RNA) (NEB E1700L), store at -20°C
- Antarctic Thermolabile UDG (NEB M0372L), store at -20°C
- dUTP solution (NEB N0459S), store at -20°C
- Magnesium sulfate (MgSO4) solution (NEB B1003S), store at -20°C
- Nuclease free water (not DEPC-treated) 100ml (Thermofisher AM9938)
Protocol
SAMPLE INACTIVATION AND LYSIS
- Thaw an aliquot of QuickExtract DNA solution on ice and vortex before use. (50µl per sample).
- On ice, mix 50µl of original sample material (nasopharyngeal swab, gargle or saliva) with 50 µl of QuickExtract solution in PCR strips or in a 96-well plate; seal the plate (or close the PCR strips with the lids).
- Heat-inactivate QuickExtract:Sample mix at 95°C for 5 minutes, then cool on ice or store at -20°C
[PAUSE] Sample can be stored at -20°C at this point for up to four weeks, potentially longer. Inactivated samples can now be handled in BSL-1 settings.
rt-lamp reaction
Calculate your reagent consumption according to this table
| Reagent | Per 10 ul reaction (μl) | Final concentration |
|---|---|---|
| WarmStart LAMP Kit (2x) | 5 | 1X |
| Primer mix (10x) | 1 | 1X |
| HNB Dye solution (3 mM) | 0.4 | 120 μM |
| dUTP (100 mM) | 0.07 | 0.7 mM |
| Magnesium sulfate (100 mM) | 0.07 | 0.7 mM |
| 50x NEB LAMP Dye (optional) | 0.2 | 1X |
| Thermolabile UDG (optional) | 0.2 | 0.02 U/μl |
| WarmStart Bst2.0 DNA polymerase (8000 U/ml) | 0.4 | 0.32 U/μl |
| Nuclease free water | to a total of 8 |
- Prepare the RT-LAMP reaction mix by mixing the following reagents in the given order (top to bottom) and quickly vortex and spin down in a microfuge.
- Dispense the reaction mix into PCR strips or 96-well plate on ice, 8 µl per well (sample).
- On ice, Add 2 µl of heat-inactivated QuickExtract lysate to RT-LAMP reaction and mix carefully by pipetting the reaction up and down. Seal PCR strips by capping them, and seal 96-well plates by applying a plastic transparent plate seal.
[CRITICAL] This step should be done in a separate work area where no reagents are handled.
[CRITICAL] If performing colorimetric detection, take an image of the PCR strips or 96-well plate with the mastermix and samples. - Transfer the reaction to a suitable stable heat-source such as a heat block or a thermocycler and run reactions at 63°C for 35 minutes.
[OPTIONAL] For real-time fluorescence data acquisition in qPCR thermocycler, we typically perform 35 cycles at 63°C with 1-minute cycle length and reading at the end of each cycle. NEB fluorescent dyes require data acquisition using a standard FAM or SYBR filter (494nm/518nm absorption/emission). - After 35 minutes run-time, remove reactions and allow to cool briefly at room temperature. Then proceed to inspect reactions visually. Negative reactions are purple while positive reactions are sky-blue.
[CRITICAL] If performing colorimetric detection, take a second image of the PCR strips or 96-well plate under the same conditions as you took the first one after the reaction. - Discard the reactions immediately after noting down the results.
[CRITICAL] DO NOT OPEN THE REACTION TUBES!
Readout methods
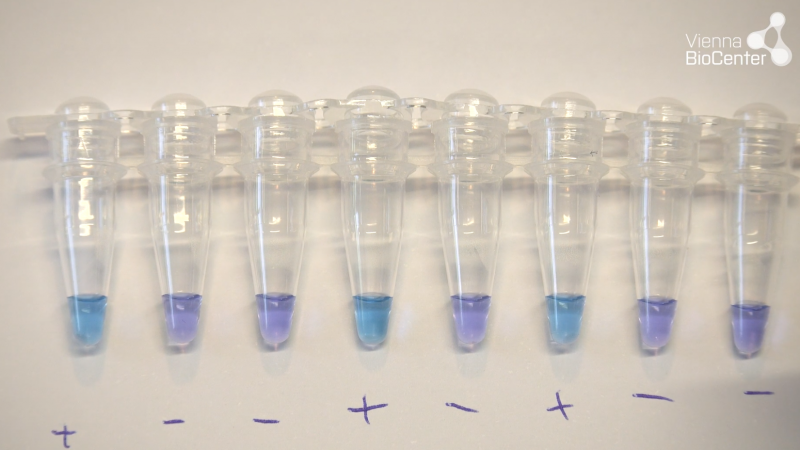
Visual colorimetric
Hydroxynaphtol blue (HNB) is used in RT-LAMP reactions that do not include any purification step. The dye changes colour from purple to sky blue upon presence of target sequence and amplification. This can be visualised with the naked eye, and reactions can be scored directly after taking them out of the heating device.

App-assisted colorimetric
For people who find the colour change of HNB difficult to see with the naked eye, we recommend the use of a simple web app. Developed by Andrew Straw to increase the colour difference between positives and negatives and make the readout much simpler. Try it out on colorimetry.net

Fluorimetric
Amplification of DNA in RT-LAMP reactions can also be read out through the use of fluorescent intercalating dyes. These dyes, such as Syto9 or the NEB LAMP dye, enable the user to either monitor the progress of the reaction in real time through the use of a qPCR machine, or end-point detection with a plate reader or a similar fluorescence detection device.
Premixing information
Assembling each master mix for every testing session from scratch is not necessary, however freezing prepared master mixes increases the risk of running into sensitivity or specificity issues. We’ve tested the possible combinations and now we can tell you what you can and can’t premix for easier reaction assembly without compromising on the quality of the tests.
LAMP reaction premix
| Reagent | Per 100 reactions (10 μl size) |
|---|---|
| WarmStart LAMP Kit (2x) | 500 |
| dUTP (100 mM) | 7 |
| Magnesium sulfate (100 mM) | 7 |
| Thermolabile UDG (optional) | 20 |
| WarmStart Bst2.0 DNA polymerase (120 000 U/ml) | 2.6 |
| total | 535 |
Add 5.35 µl of this mix per 1 reaction.
Note: A concentrated version of WarmStart Bst2.0 polymerase (NEB cat. nr. M0538M) is used to minimise the volume of the premix.
Store at -20 °C.
LAMP primer premix
| Reagent | Per 100 reactions (10 μl size) |
|---|---|
| 10x LAMP primer mix | 100 |
| nuclease-free water | 100 |
| total | 200 |
Add 2 µl of this mix per 1 reaction.
Note: You may use the As1, E1 or ACTB primers to make a LAMP primer premix. Make sure to label the mix adequately.
Store at -20 °C.
LAMP HNB premix
| Reagent | Per 100 reactions (10 μl size) |
|---|---|
| HNB Dye solution (3 mM) | 40 |
| nuclease-free water | 25 |
| total | 65 |
Add 0.65 µl of this mix per 1 reaction.
Store at 4 °C.
The premixes for commercial RT-LAMP enable you to have three tubes in your freezer and fridge and to assemble reactions easily using 5.35 µl of LAMP reaction buffer premix, 2 µl of LAMP primer premix and 0.65 µl of LAMP HNB premix per 10 µl reaction. We have observed these premixes to be stable for a month at the specified storage conditions, but they are most likely good even for longer.
